ClinPay
Participant Compensation Disbursement
Compensation is paid directly to participants electronically. The whole process is online - recorded, transparent, traceable, and with desensitized data. Sponsors can disburse subsidies safely & compliantly.
It lightens hospitals' workload by cutting out redundant steps. A tailored session will be arranged to showcase platform capabilities most relevant to your clinical development priorities.
Product Advantages
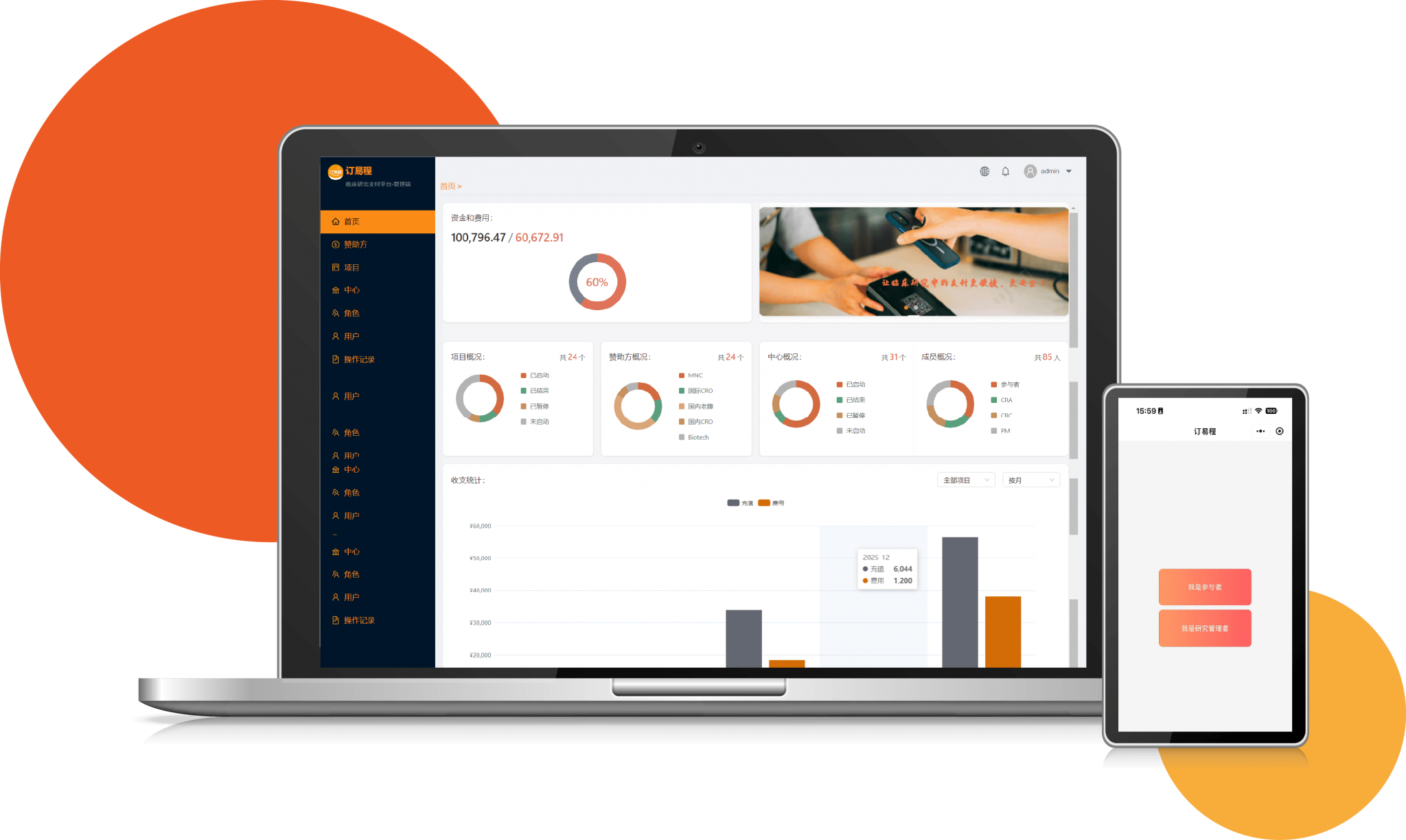
Our Clinpay system records all clinical research transactions online, ensuring full transparency, traceability, and compliance with medical financial norms for trusted multi-party cooperation.

Post one-time contract signing, standardized settlement documents meet global clinical standards and local regulations, boasting legal validity and easy retrieval to eliminate compliance risks.

End-to-end systemized operations cut manual intervention, enabling fast fund recharge and prompt subsidy disbursement to optimize participant experience and trial progress.

Adhering to HIPAA/GDPR, we use bank-level encryption to protect patient privacy, fully safeguarding participants’ legitimate rights throughout the clinical research journey.

